
There are two different types of solar panel, both of which are
used to make electricity. Both use the energy from the Sun - an
effectively inexhaustible supply - to generate electricity for our
needs. SOHO (and many other satellites, like the Hubble Space
Telescope (launched from the Space Shuttle) pictured below) use solar
panels as a way of generating the electricity they need to
operate.


|
The first type uses the Sun's heat energy to vaporise a liquid. The gas of this liquid is then used to drive a turbine (do you know how a turbine works?) which generates electricity. This type of solar panel can be used to generate electricity for your home. SOHO does NOT have this type of solar panel - can you give a reason why?. |
| SOHO uses the second type of solar panel, called photovoltaic. The word "photovoltaic" comes from "photo", meaning light and voltaic, an adjective that relates to producing electricity. |
| Here's how it works. First of all we need to know a little bit about light rays. Instead of thinking of a light ray as being a wave, it sometimes useful to think of a light ray as being a particle instead. We call these particles of sunlight photons. (Why do we have to think of light ray as being either a wave or a particle? Well, to be honest, no scientist really knows what a light ray looks like. Sometimes a light ray behaves as if it were a wave, and sometimes it behaves just like a particle. It's like that, and that's just the way it is. So it helps us to call a light ray either a wave or a particle.) |

|
|
| Each photon carries a very specific amount of energy dependent on the frequency - you can think of it as a little packet of energy riding on a wave with a particular frequency. Red light photons have less energy than blue light photons because the frequency of red light is less than that of blue light. |
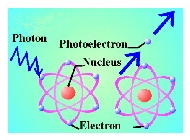
| What happens when a photon hits something solid, like a metal, or an atom of silicon, say? We'll stick with silicon beacuse that is what photovoltaic solar panels are made of. |
| Well, as you know, atoms consist of a dense positively charged nucleus, a whole lot of empty space and a bunch of negatively charged electrons. Each of these electrons sits in a particular energy level. Let's now imagine a photon interacting with an electron. The electron will absorb the energy of the photon. Therefore, the electron now has more energy than it had before, so it has to change it's energy level. If the original photon had enough energy the electron now has sufficient energy to leave the atom of silicon completely. When this happens, this is called the photoelectric effect. |
|

|

|
| Frequency too low - no electrons | Frequency high enough - electrons |
| Solar panels are made so that when the electron leaves the atom it crosses a one-way junction and then cannot return. Since we have a postively charged nucleus and a negatively charged electron separated from each other, we have created a potential difference. This can be used to drive an electric current round a circuit and what do we get? - electricity from sunlight! Enough to let you drive a car.... |

Use the browser's BACK button
to return to the previous page.