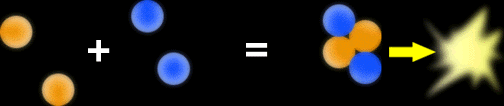Kelvin and the Sun
The source of energy for the Sun was a major concern for scientists last century. Lord Kelvin (born William Thomson, picture on the right) thought that the Sun was powered by the conversion of gravitational potential energy into heat and light. Given the size and mass of the Sun he calculated that the Sun was about 50 million years old.Now this was a problem since the Earth was calculated to be at least 400 million years old! It hardly seemed possible that the Earth could be older than the Sun. Incidentally, both the Sun and the Earth are now thought to be around 4.5 billion years old.