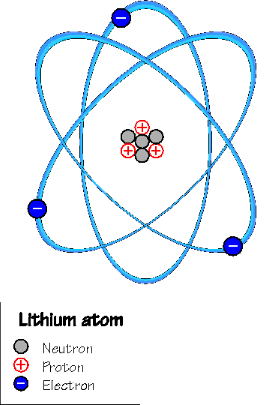
The nucleus contains neutrons and protons, which both have the same mass , but neutrons are electrically neutral, whereas protons have a positive charge. The electrons have very little mass and are negatively charged.
Since an atom contains the same number of electrons as it does protons, it is electrically neutral. However, it is quite easy for an atom to lose an electron, and then it's called an ion. The important thing about an ion is that it has an electric charge (positive), which makes it behave very differently from atoms. A gas that contains ions is called a plasma.