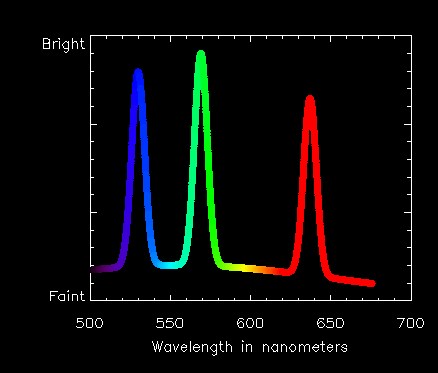

Extra brightness at these wavelengths is produced by atoms of iron (Fe) and calcium (Ca) in the solar atmosphere.
But they are not ordinary atoms of iron and calcium. They are ions of those elements - atoms that have lost many of their electrons.
In the case of the line at 530.3 nanometers, it is produced by iron atoms which have lost 13 of their 26 electrons.
The 569.4 nm line is from calcium atoms that have lost 14 of their electrons.
The 637.4 nm line is again from iron, but you might say these atoms have had a less traumatic time and have 'only' lost 9 electrons!
But why do we see different ions of iron if we are looking at the same piece of the Sun's atmosphere? That is telling us that that part of the atmosphere contains material at different temperatures. For many reasons the Sun's atmosphere is not stirred like a cup of coffee so that it all has the same temperature (even if you put milk in). It's almost as if when you put the milk into coffee the milk stays cool and the coffee stays hot - not at all what you'd expect! Part of my work is aimed at finding out how this happens.
Use the BACK
button to return to the
Finger Prints of the Sun