There are three main features seen in spectra:
The background light is smooth, called the continuum.
In an atom, the electrons orbit around the nucleus at set distances. They cannot go anywhere they like. When an electron jumps to a smaller orbit it gives out a photon of radiation, an emission line.
When an electron wants to go from a smaller orbit to a larger one, it needs energy. It takes energy away from the light which is passing through it - so we see an absorption line.
| Did you know? The element Helium was discovered using the light from the Sun (and was named after the Greek Sun God). At the time, in 1868, there was no known material on Earth which could produce a spectral line at the same wavelength. Helium was finally discovered on Earth some 30 years later. |
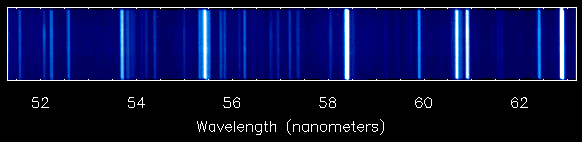
Here is a spectrum from SOHO showing an emission line spectrum from the corona in the ultraviolet
