
Photo: Rich Thompson
You must have seen a rainbow sometime in your life. You have probably learned that this happens when the raindrops split the Sun's light into its different colours or "wavelengths". We call this a spectrum (the plural is spectra).

The simplest way to produce a spectrum in school is using a prism.

Courtesy of the YOHKOH public outreach page
Astronomers cannot always wait for 'sunshine and showers', so they build special instruments for measuring the spectra of light, called spectrometers. With these instruments they are not limited to studying the wavelengths of light we can see with our eyes. Astronomers can study longer (redder) and shorter (bluer) wavelengths. Beyond the blue end of the visible spectrum is the ultraviolet (UV). This is what our skin reacts to when we get a tan and is the light which gives us a lot of information about the Sun.
When astronomers first looked at the spectrum from the corona during an eclipse they saw a strange effect. Instead of the smooth appearance of a rainbow, the brightness (intensity) of light varied a lot at different wavelengths. This is a plot of the kind of spectrum they saw. We have just plotted how bright the light is at different wavelengths.
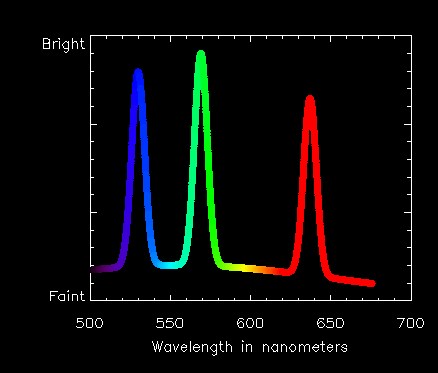
We call these special brightenings "spectral lines" in the solar spectrum.
At first no one could figure out how they were produced. Scientists knew that atoms or ions emit light at their own very particular wavelengths. The spectral lines are like fingerprints which tell us which "ions" are present in the gas.
| BUT (yep that's a big but) no one had ever seen spectral lines at these wavelengths before, so they hadn't a clue which "ions" they came from. So what did they do? They cheated! They invented a new element called CORONIUM to explain the brightenings. A bit like creating Frankenstein to solve a murder mystery! |

|
Eventually, the mystery was solved and the fingerprints were found to belong to ions which were very, very hot.
The extra brightness at these wavelengths is produced by ions of iron (Fe) and calcium (Ca) in the solar atmosphere.
In the case of the line at 530.3 nanometers, it is produced by iron atoms which have lost 13 of their 26 electrons. The 569.4 nm line is from calcium atoms that have lost 14 of their electrons. The 637.4 nm line is again from iron, but you might say these atoms have had a less traumatic time and have 'only' lost 9 electrons!
In order to produce these ions, they knew that the solar corona must be very hot, very hot. They come from a gas at a temperature of about two million degrees, hotter than anything on Earth. So, it is not surprising that this puzzle was so tricky to solve.


|